Applicant
Leiter Forschung und Entwicklung
Klinik für Unfallchirurgie, Orthopädie und Plastische Chirurgie
Universitätsmedizin Göttingen
Robert-Koch-Str. 40
37099 Göttingen
Telefon (Sekretariat): +49 551 3922462
direct contact: +49 551 398989
E-Mail: arndt.schilling@med.uni-goettingen.de
Cartilage Adjacent Subchondral Bone (CASB) in ageing and disease as a diagnostic and therapeutic target

Careful evaluation of animal experimental data of osteoarthritis (OA) has revealed that changes in the subchondral bone structure precede the loss of cartilage. These results suggest that the increased bone mass in the subchondral zone may also be a cause rather than only an effect of OA. In this project we will specifically study at ultra-high resolution the zone between the cartilage and the spongy bone consisting of tidemark, calcified cartilage and subchondral bone, which we will term cartilage adjacent subchondral bone (CASB). In healthy joints, this complicated structure is reported to gradually relay impacting forces from the soft cartilage to the hard spongy bone.
Our working hypothesis is that the three-dimensional nano-architecture of the cartilage adjacent subchondral bone (CASB), changes during the development of OA. We further hypothesize that these changes play an important role in the development of osteoarthritis and can be utilized for the development of early diagnostic tools, for the monitoring of treatment and the development of novel therapeutic strategies.

Consequently, our main objective is it to investigate the 3D-nano-architecture of the CASB in healthy and osteoarthritic samples, correlate observed changes in the bone to changes in the cartilage and develop theories to explain our findings and propose novel diagnostics and treatment modalities.
To reach these objectives we will follow 6 specific aims: We will study the physiologic development of the nano-architecture of the CASB in animal models (Aim 1). New tools will be developed to enhance visualization and analysis of CASB 3D-data through 3D-printing and Virtual Reality (VR) imaging (Aim 2). Changes in the nano-architecture of the CASB will be analyzed in animal models of OA-pathology (Aim 3). The CASB will be studied in human samples of OA (Aim 4). Based on the data collected in Aim 1-4 we will evaluate diagnostic and treatment options (Aim 5) and will develop a new model of cartilage-CASB interaction (Aim 6).
Key Achievements of the First Funding Period
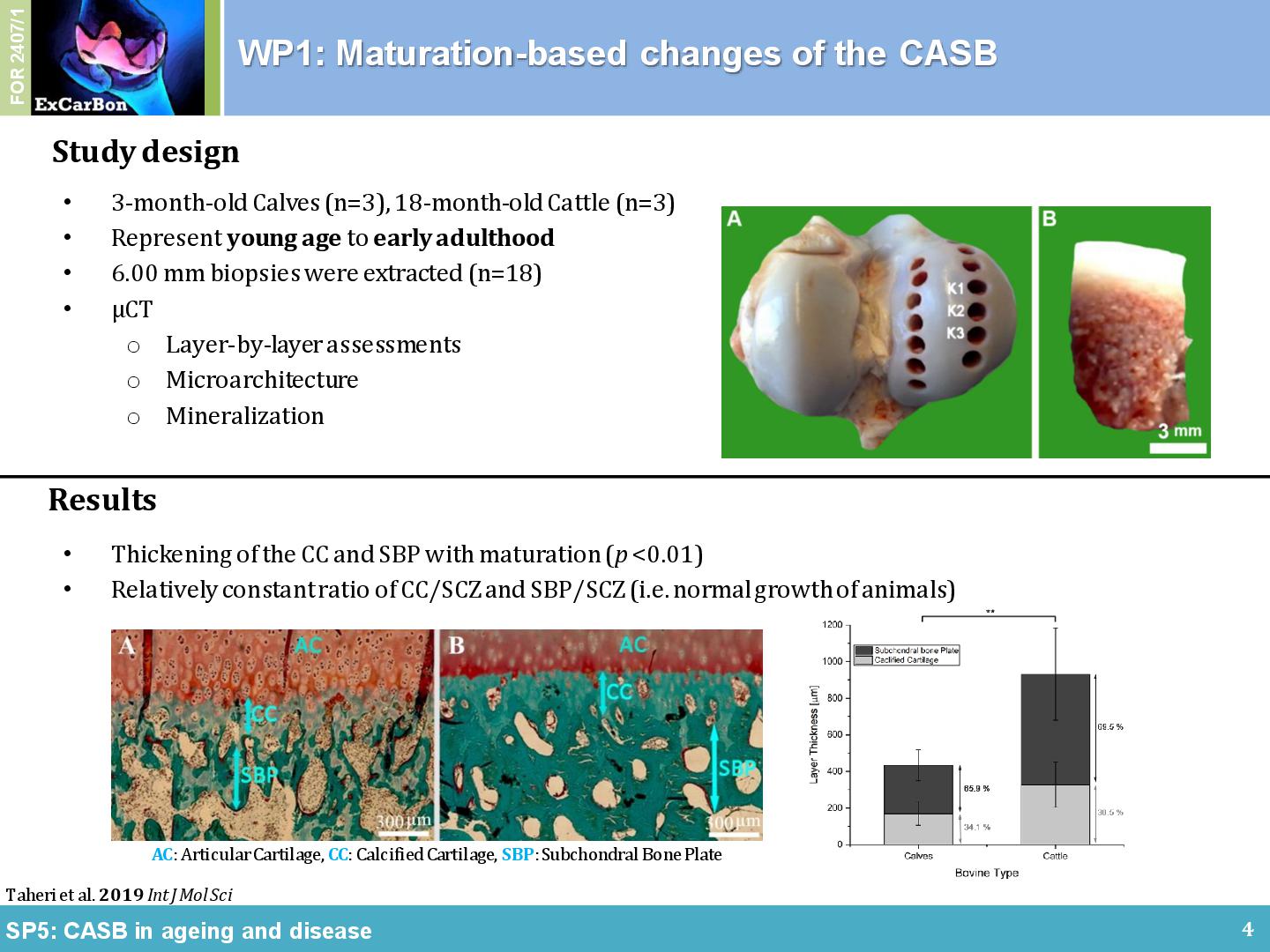
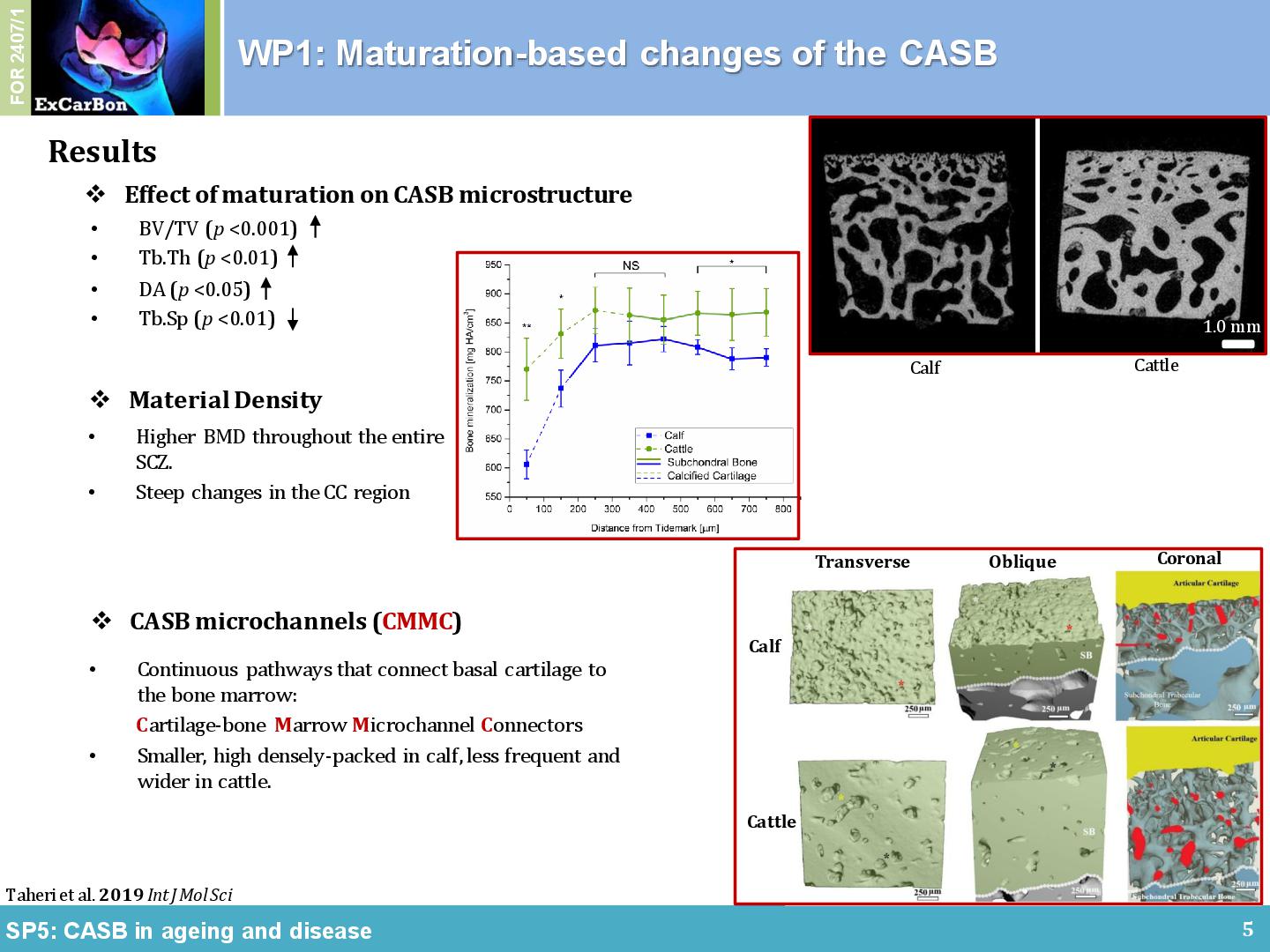
The non-calcified articular cartilage (AC), the calcified cartilage (CC) and the cortical and trabecular subchondral bone (SB) in the joint form a bio-composite that is uniquely adapted for load dissipation. Within ExCarBon, we have hypothesized that the calcified cartilage and the SB, termed cartilage adjacent subchondral bone (CASB), harbors a distinct 3D-microarchitecture that is correlated with the overlying cartilage during physiological joint development, OA initiation and progression (SP5). As supporting evidence, we have demonstrated a developmental transformation of connective cavities within the CASB of the medial femoral condyle during bovine joint maturation using high-resolution nanoCT, histomorphometry and scanning electron element analysis [1]. The maturation of healthy bovine subchondral bone is primarily dictated by an increase in BV/TV caused by trabeculae thickening (Figure 1A). In comparison with the distance from the tidemark, maturation has less impact on the number and the connectivity of the trabeculae. Calcified cartilage may be considered as a prime focus for future examinations owing to its steep mineralization profile and dynamic composition characteristics (Figure 1B). It was also found that the material properties of the surrounding bone changed in relation to distance from the tidemark (Figure 1B). A series of intricate microchannel structures connectead the subchondral trabecular bone to the tidemark (i.e. CMMC), which were more frequent and smaller in young ages while less frequent and enlarged in early adulthood (Figure 1C). Owing to this apparent age-dependent morphology and interconnection between medulla and articular cartilage, CMMCs may play a pivotal role in the (patho)physiology of the joint, hence, the changes of the microchannel network in correlation with AC thickness and other hallmark features of OA were particularly interesting as focal points for further investigations.
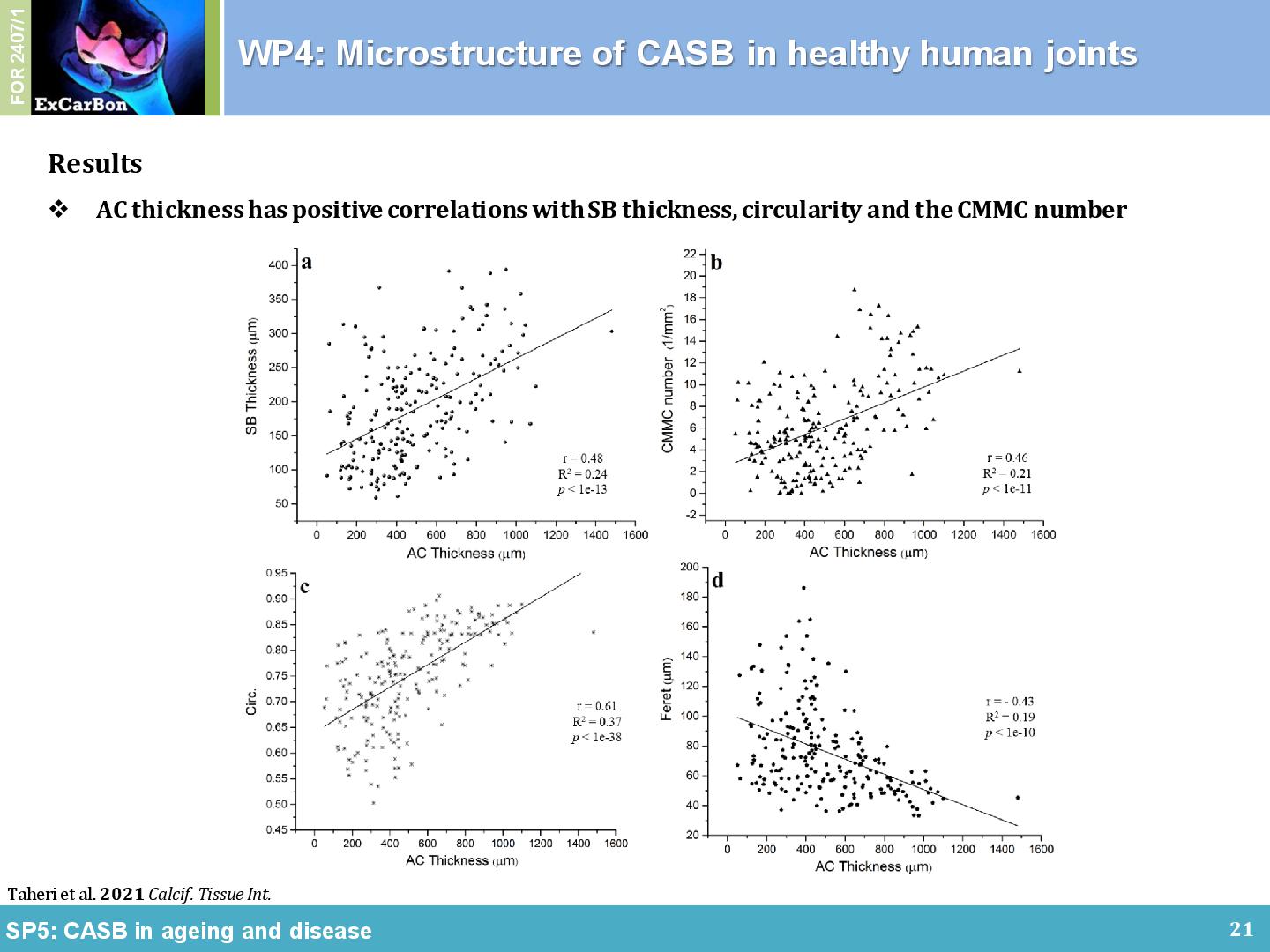
Our previous methodology in high resolution imaging was adapted to create and quantify an unprecedentedly detailed 3D map of the CASB microarchitecture in healthy human femoral heads. Samples collected and graded according to the Outerbridge classification by orthopaedic surgeons and then analyzed according to their physiological biomechanical loading. By profiling the CMMC in normal physiological human femoral heads, a strong association with loading areas was found. In general, the CMMC were small, circular, and with high local density in the LBR (Figure 2A), while intermittent, irregularly-shaped, and significantly enlarged in the non-load-bearing region (Figure 2B) and the peripheral rim (Figure 2C) of the joint [2]. Cartilage thickness was positively associated with CASB thickness, CMMC number, and circularity index. In conclusion, our data suggest that CASB microarchitecture is even more intricate than originally thought, and that regional differences in the microchannel architecture of CASB might reflect regional differences in loading. It is proposed that the textbook models of cartilage-bone interface, which consider CASB as an impermeable layer, should be revised in order to incorporate CASB microchannels.
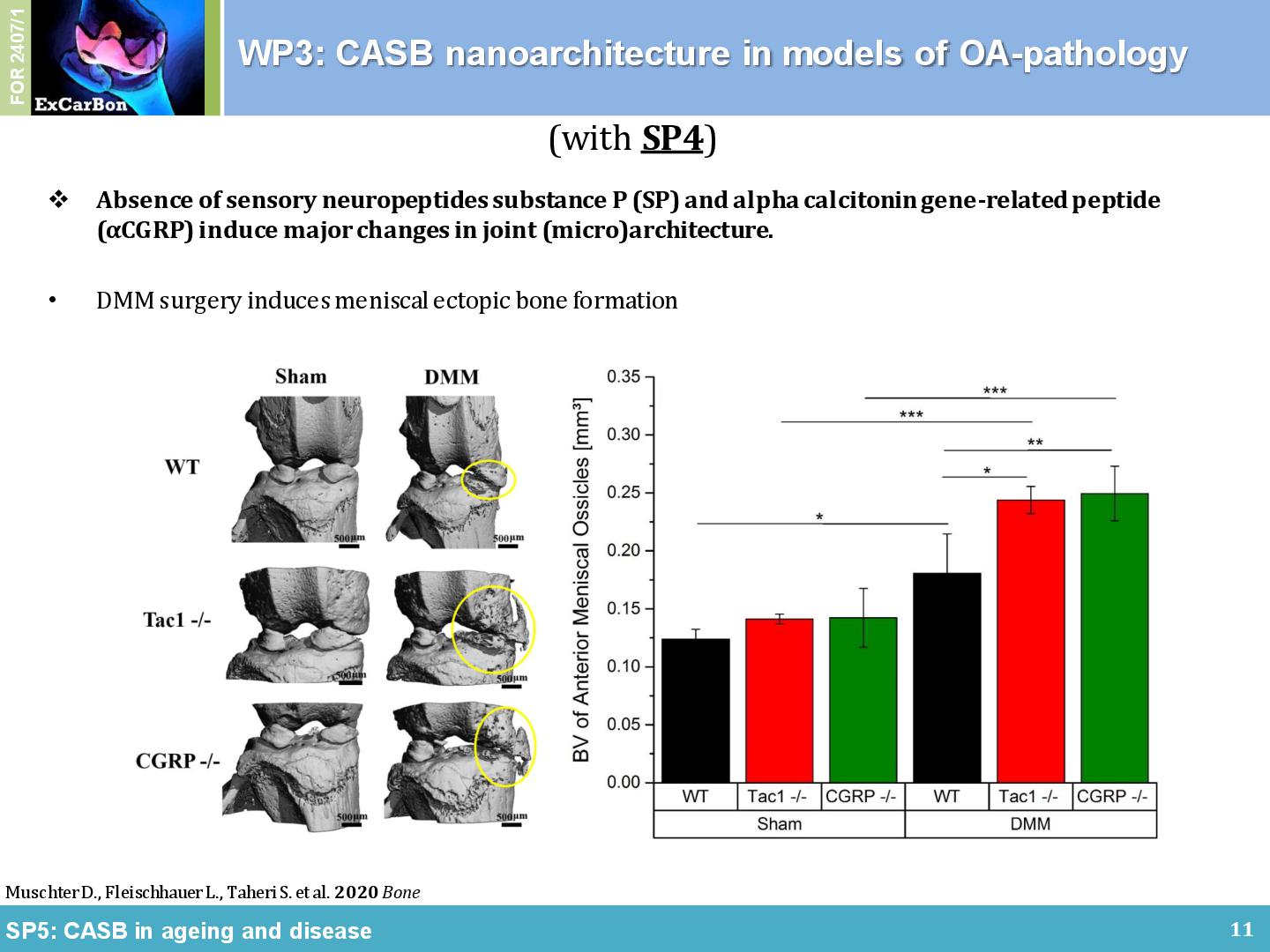
Together with the partners from the consortium we could show a contribution of the CASB in several animal models of OA. We found a subchondral bone phenotype in Ndst1-flox / transgenic Col2-Cre mice (SP2), to be precise, an angulation of the tibial tubercle in the calcified cartilage (CC) layer compared to the wildtype mice (Figure 3A). Together with SP4, we evaluated the role of sensory neuropeptides on structural alterations of bone and cartilage during murine OA development in DMM models. 2 weeks after surgery, we observed no differences in the BV/TV of the medial epiphysis. With progression of OA until 8 weeks after surgery, however, osteophytes developed in all models after DMM (Figure 3B, marked by asterisk) and significant subchondral bone sclerosis was detected. We also found that the DMM mice developed ectopic bone at the impact side increasing the volume of the meniscal ossicles. 8 weeks after the DMM surgery, BV of ossicles from Tac1-/- and αCGRP-/- mice increased significantly compared to WT mice, and in all three mouse groups meniscal ossicle formation was stronger in DMM mice compared to Sham mice [3].
Together with SP7, we studied the role of mesenchymal stem cells (MSCs) on the healing of CASB in an early rabbit OA model. Artificial defects were created by dental drills on the medial condyle of the subchondral bone in SP7, while lateral condyles served as control groups. 12 weeks after generating artificial defects by dental drills, new bone formation was assessed under different oxygen concentration levels (hyperoxia vs. physioxia), and with or without treatment. We found similar bone generation under hyperoxia and physioxia (51% and 45%, respectively) when compared to intact lateral condyle, while bone generation with pure hydrogel filled into the defects (without MSC treatment) was only 19% after 12 weeks (Figure 3C).
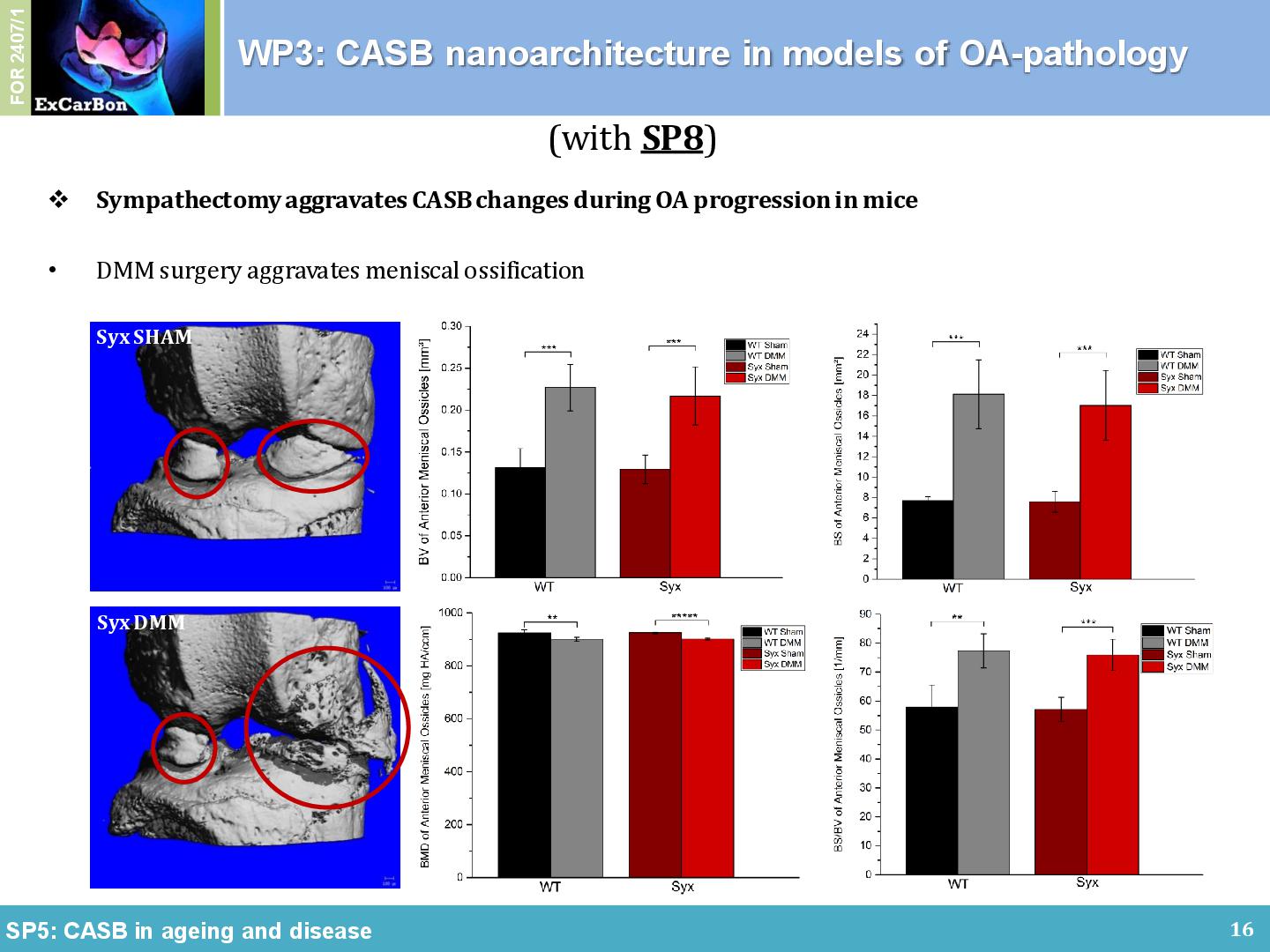
It is reported that the most prominent sympathetic neurotransmitter norepinephrine has been detected in the synovial fluid of trauma and OA patients. Hence, together with Jenei-Lanzl/Straub (SP8) we analyzed how sympathicus influenced the CASB development in an in vivo model. We observed that all DMM-operated mice showed osteophyte formation at the medial margins of the knee joint, which led to an increase of the diameter of the medial tibial plateau. There was a decrease of the medial condyle diameter in sympathectomized mice (Syx) compared to C57BL/6 wildtype mice (WT), an increase of the CC thickness (Figure 3B), an increase of SCBP thickness in the medial condyle after DMM surgery, and an increase volume size of the meniscal ossicles. Overall, we found that Syx attenuated cartilage degradation but aggravated OA-specific subchondral bone changes, suggesting independent pathways of the two pathologies.
[1] Taheri S, Winkler T, Schenk LS, Neuerburg C, Baumbach SF, Zustin J, Lehmann W, Schilling AF. Developmental Transformation and Reduction of Connective Cavities within the Subchondral Bone. Int J Mol Sci. 2019 Feb 12;20(3):770. doi: 10.3390/ijms20030770.
[2] Taheri S, Yoshida T, Böker KO, Foerster RH, Jochim L, Flux AL, Grosskopf B, Lehmann W, Schilling AF. Investigating the Microchannel Architectures Inside the Subchondral Bone in Relation to Estimated Hip Reaction Forces on the Human Femoral Head. Calcif Tissue Int. 2021 Nov;109(5):510-524. doi: 10.1007/s00223-021-00864-x.
[3] Muschter D, Fleischhauer L, Taheri S, Schilling AF, Clausen-Schaumann H, Grässel S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone. 2020 Apr;133:115181. doi: 10.1016/j.bone.2019.115181.