Applicant
Name: Prof. Dr. Peter Angele, M.D., Ph.D.
E-Mail: peter.angele@ukr.de
Portrait: www.sporthopaedicum.de/fachaerzte/prof-dr-peter-angele/kurzportrait.html

Preconditioning of mesenchymal stem cells with mechanobiological load and hypoxia for joint regeneration in moderate osteoarthritis
The presence of osteoarthritic changes in a joint is a contraindication for current tissue engineering approaches, which are designed for regeneration of localized traumatic osteochondral lesions. Our long-term goal is the restoration of degenerative osteochondral lesions and beyond that, the treatment of chondral and osteochondral defects in an OA environment. To be successful we need to understand the constructive and destructive interactions between chondrogenesis, mechanical stress, oxygen tension and inflammation.
We propose that mesenchymal stem cells can be preconditioned with mechanobiological load and/or hypoxia prior to implantation in order to achieve a stable chondrogenic phenotype. This preconditioning could allow regeneration of osteochondral defects even in an OA environment.
Mechanical load in physiological range is an important factor for maintenance of the healthy status of joint cartilage. On the other hand, overloading is believed to increase the risk of osteoarthritis. Therefore we will apply hydrostatic pressure in different mechanobiological loading regimes to MSCs undergoing chondrogenesis in an in vitro 3D-aggregate culture system. We will identify loading conditions that show the strongest chondrogenic (increased anabolism and decreased catabolism) difference between loaded and unloaded conditions. We plan to achieve this under physiological conditions, but also in OA conditions, which will be mimicked by addition of IL-1ß to the culture medium.
Hypoxic culture conditions have also been shown to have beneficial preconditioning for chondrogenic cells. We propose that hypoxia will promote differentiation and suppress markers of hypertrophy in both healthy and osteoarthritic culture conditions. We will apply hypoxia to MSCs undergoing in vitro chondrogenesis and analyse for anabolic and catabolic effects. We will focus on the suppression of hypertrophy in order to achieve a stable chondrogenic phenotype. We plan to identify key signaling pathways involved in the preconditioning process. In particular, we will analyse the PI3K/Akt-dependent pathway, because this cascade is involved in the modulation of chondrogenesis through mechanotransduction and also hypoxia.
Finally we want to examine the effect of MSC-sponge constructs, preconditionend with mechanobiological load and/or hypoxia, to repair posttraumatic osteochondral defects in an animal model in a healthy and an early osteoarthritic condition.

Hydrostatic pressure device for stimulation of MSC chondrogenesis
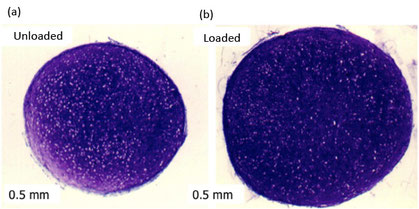
Mesenchymal stem cell (MSC) chondrogenesis following 21 days differentiation either (a) unloaded or (b) after 7 days loading under hydrostatic pressure during first week of culture. Stained with DMMB for the presence of sulphated glycosaminoglycans

(a) Phosphotidylinositol-3-kinase (PI3K) as a signalling molecule: Mechanical stimulation or growth factor binding activates the dimerization and phosphorylation of the receptor monomers. A membrane-bound GTP and Ras form a complex, which, in turn, activates PI3K. The resulting effect shows the activation of Akt, also known as protein kinase B. (b) Interactions between different signaling molecules with special focus on mechanotransduction-dependent pathways
Mercator Fellowship of Prof. Dr. Brian Johnstone, Visiting Professor at the Department of Trauma Surgery, University Regensburg Medical Centre
Thematically the Mercator Fellowship is focusing on examination of MSC chondrogenesis under hypoxia and specifically its effect on Hypoxia Inducible Factors (HIFs). Prof. Dr. Johnstone will have three periodical visits during his Mercator fellowship, including an extended three months stay in the lab. He will advise the team on setting up MSC hypoxia culture protocols and validations. Furthermore, he will also visit the other consortium members to develop collaborations within this theme.
1st Period of the Mercator Fellowship
Prof. Dr. Johnstone visited the laboratory between 16.10.16 – 16.01.17. The exchange involved consulting on various techniques concerning in vitro chondrogenesis, particularly under hypoxic culture, and on the establishment of a rabbit in vivo model of early OA for evaluation of the preconditioned cells. During his visit, he was a keynote speaker and attendee in the ExCarBon meeting at the TIRM Conference (25.11.16-26.11.16 in Regensburg) involving all consortium members, whereby he was involved in discussions regarding the project and collaborations between members that were outlined in the proposal. He also gave two lectures: 1) at the ZMB, entitled “Regeneration potential in an early OA situation from a basic research view”, and 2) at the University Hospital of Regensburg, entitled, “Stem cell tissue engineering: The good, the bad and the ugly”.
Key Achievements of the First Funding Period
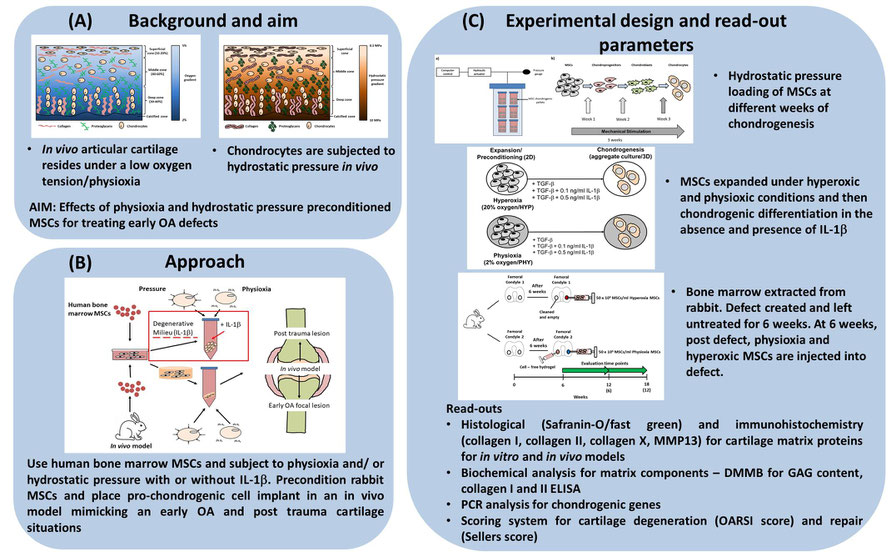
Figure 1. Schematic diagram describing the (A) background, (B) approach and (c) experimental design for SP7
Treatment of traumatic and osteoarthritic lesions of the knee joint is a major challenge in the orthopaedic field. The gold standard therapy for these defects is Autologous Chondrocyte Implantation (ACI). However, long-term follow up of early OA patients treated with ACI results in a 2-fold failure rate due to the presence of inflammatory cytokines, e.g. interleukin-1beta. Thus, alternative cell sources are required for this situation. Mesenchymal stem cell (MSC)-dependent repair of early OA defects is a promising therapeutic approach, based upon their chondrogenic potential. To facilitate the formation of a stable chondrocyte phenotype and tissue formation, an appropriate combination of environmental stimuli (e.g.mechanical and chemical factors) is required. In SP7, we have hypothesised that human MSCs derived from the bone marrow, preconditioned with a combination of mechanical load (hydrostatic pressure) and low oxygen tension (physioxia), would enable efficient repair of osteochondral defects even in an OA environment.
Hydrostatic pressure involves fluid pressurisation of fluid within the matrix without cellular deformation. Cartilage is subjected to pressure during motion in combination with compression and shear forces (Figure 1A) (1). Previous studies have demonstrated that hydrostatic pressure alone promotes MSC chondrogenic matrix production. During this first period, we observed that cyclic hydrostatic pressure loading for seven days during the second week of MSC pellet chondrogenesis leads to a significant increase in GAG content and cartilage matrix formation (Figure 2A).
Articular chondrocytes reside in vivo at an oxygen tension between 2-7% oxygen or physioxia (Figure 1A) (2). In vitro, physioxic MSC chondrogenesis has resulted in an increase in cartilage matrix proteins and suppression in hypertrophic markers. We established an in vitro cytokine-induced degenerative model through application of IL-1β and demonstrated a clear dose-dependent inhibition of MSC chondrogenesis in a pellet culture model. Interestingly, culturing MSC pellets in physioxia (2% oxygen), significantly enhanced cartilage matrix production and alleviated the inhibitory effect of IL-1β (Figure 2B).
To test the feasibility of our in vitro results in a clinically relevant situation, we evaluated our preconditioned MSCs in an animal model. We have developed a focal early OA model in rabbits by conducting highly standardized drilled osteochondral defects. Using this model, we showed that transplantation of physioxia preconditioned rabbit bone marrow MSCs resulted in greater tissue integration, de novo cartilage formation and overall better defect repair compared to hyperoxic (20% oxygen) conditioned rabbit MSCs (Figure 2C).

Figure 2. (A) Macroscopic images of unloaded and week 2 loaded MSC chondrogenic pellets at day 21 with total wet weight of MSCs chondrogenic pellets on day 21 (Data represent mean + S.D; *p < 0.05). (B) Pellet wet weight in the presence of IL-1b and under hyperoxic (20% oxygen) and physioxic (2% oxygen) conditions (Data represent mean + S.D.; *p < 0.05) with representative macroscopic images. (C) Representative images of hydrogel only, physioxic MSCs and hyperoxic MSCs treated early OA defects and the Sellers score indicate the cartilage repair in untreated and treated defects (Data represent mean + S.D.; *p < 0.05)
References
1) Pattappa G, Johnstone B, Zellner J, Docheva D, Angele P. The importance of physioxia in Mesenchymal Stem Cell chondrogenesis and the mechanisms controlling its response. Int J Mol Sci. 2019 Jan 23;20(3).
2) Pattappa G, Zellner J, Johnstone B, Docheva D, Angele P. Cells under pressure - the relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. Eur Cell Mater. 2019 May 6;37:360-381
